We’re very proud to be working with Professor Mark Faghy at the University of Derby. His ground-breaking ERASE-LC trial, the UK’s first Long Covid antiviral drug study, is shedding new light on post-viral recovery.
Mark and his team are exploring whether a treatment first used in acute COVID can support people still living with ongoing symptoms from COVID, and how Visible can help measure recovery.
We spoke to Mark about his research and the role Visible is playing in the ERASE-LC trial.
How did you come to study Long Covid?
Before the pandemic, our research looked at how people recover from pneumonia. We were trying to understand why some patients still felt unwell weeks after being told they were “clinically recovered.” We realised there was a slower, under-recognised stage of recovery that wasn’t being addressed.
When COVID emerged, I was preparing a major funding application for this work. The call was cancelled, but we were able to quickly adapt our research framework to focus on what became known as Long Covid. Since then, our team has carried out several projects to understand what causes ongoing symptoms and how we can better support people with Long Covid.
What is your research trying to find out?
Our ERASE-LC trial is studying why some people don’t fully recover after COVID and whether the antiviral drug Remdesivir might help. The research examines the immune system, inflammation and other changes in the body to understand the causes of ongoing symptoms and whether antiviral treatment could support recovery.
How does Visible help you do your research?
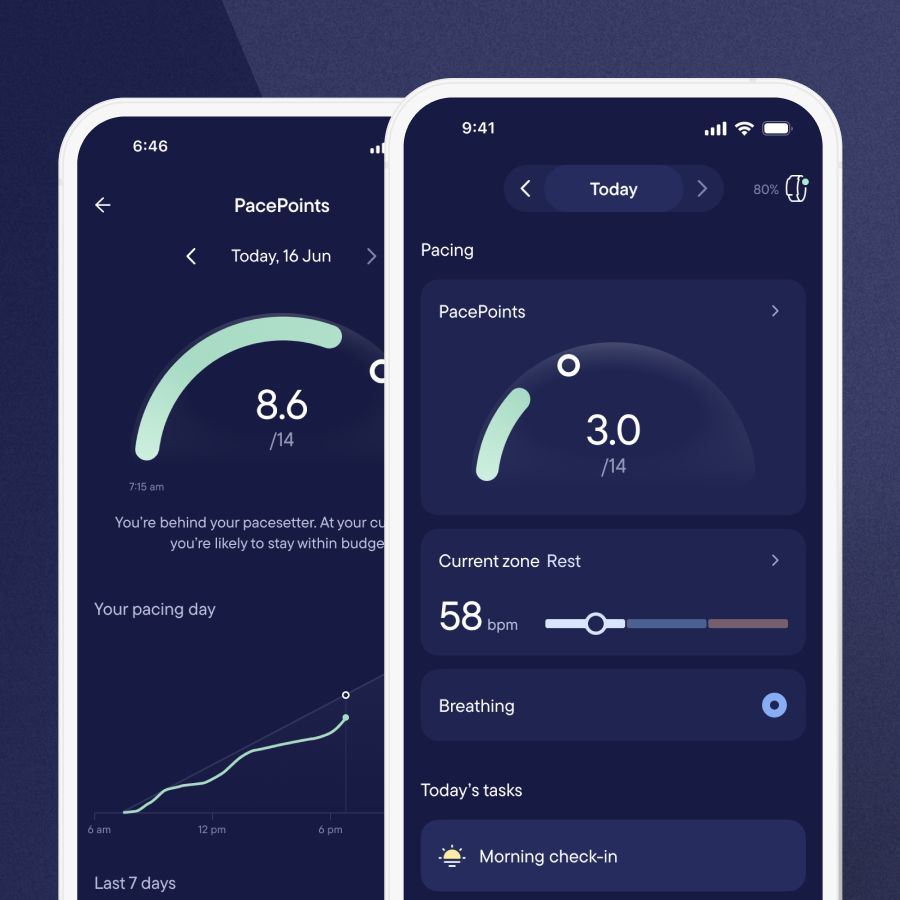
We are collecting an extensive range of data, from blood samples and symptom questionnaires to physiologic assessments and functional tests, to build a complete picture of participants’ condition before and after treatment.
However, traditional testing can’t always capture the reality of living with Long Covid. Many patients conserve energy before appointments, meaning lab data might not reflect daily experience. That’s where Visible can play a crucial role. Participants wear Visible for more than 50 days, providing continuous real-world data on heart rate, variability and symptom patterns.
“Traditional tests give us a snapshot, not the full picture of how someone functions day-to-day,” explains Professor Faghy. “That’s where tools like Visible make such a difference, they let us see what’s happening between clinic visits, in the patient’s real world.”
Who are you collaborating with on this study?
This project is incredibly collaborative. We’re working with colleagues at University Hospitals Derby and Burton, University of Plymouth and the University of Exeter, and a network of national and international partners.
All of our research is heavily shaped by continual input and engagement from people with lived experience. Patients have been involved at every stage of the process, from shaping the study design to defining what meaningful recovery looks like. That collaboration ensures we’re focusing on outcomes that matter most to people with Long Covid.
The philosophy is simple: share openly, talk to everyone and don’t hold secrets. Complex problems like Long Covid need true interdisciplinary, collaborative and transparent approaches.
What’s next for your research?
Right now, we’re in the analysis phase of ERASE-LC, looking for signs of physiological change and markers of improvement. If the signals are positive, we’ll move toward a larger, randomized controlled trial. We’re preparing a major grant application, due in December, to scale up this work.
Beyond that, I’m passionate about refining how we measure recovery. Traditional lab metrics don’t always capture what patients experience. We need to develop tools and frameworks that connect scientific data with lived experience, where technology like Visible will continue to play an essential role.
What motivates you in this work?
The patients. Without question. I’ve had people stop me in the street years after participating in earlier studies to tell me how much it meant that someone listened. There’s a lot of frustration in this field - political resistance, funding hurdles - but I’m stubborn. I believe we can and have to find answers if we keep collaborating and stay grounded in what patients tell us.